300 Years of Element Discovery in 99 Seconds
Chemical elements are the building blocks of modern society.
Our fundamental understanding of the periodic table has allowed us to: build rockets that can withstand scorching temperatures; harness permanent magnets that can help us generate electricity; erect ultra strong and tall skyscrapers; and discover compounds that can eradicate disease around the world.
But while we take this elemental knowledge for granted today, there was a time not too long ago when the periodic table was mostly empty.
The Elemental Dark Age
Today’s animation comes to us from materials scientist Dr. Jamie Gallagher and it chronicles the last three centuries of discoveries for the periodic table of elements.
It starts in the year 1718, around time of Isaac Newton, when the scientific method was young and the knowledge we had around chemistry was still very incomplete.
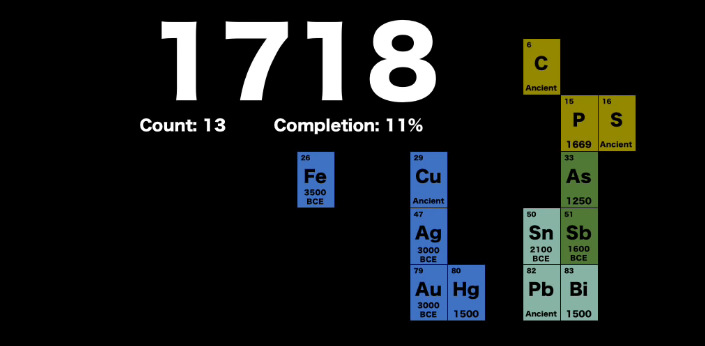
At the time, we knew about elements like iron, copper, gold, silver, and lead – but the periodic table contained just 11% of elements compared to today.
A Flurry of New Discoveries
In the late 18th century and early 19th century, researchers started seeing patterns that allowed them to make new discoveries.
Specifically, the years between 1788-1825 were particularly fruitful – over this stretch, the periodic table more than doubled in size from 26 to 53 elements.
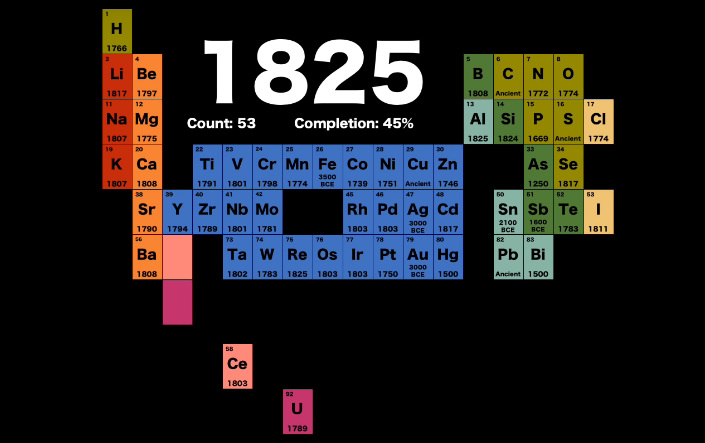
Lithium, calcium, titanium, vanadium, tungsten, palladium, silicon, niobium, and uranium were some of the elements to join the table during this critical time period.
Formation of the Periodic Table
In the 19th century, the French geologist Alexandre-Emile Béguyer de Chancourtois was the first to notice the periodicity of elements, and in 1862 devised an early version of the periodic table.
A few years later, in 1869, Russian chemist Dmitri Mendeleev created a table organized by atomic mass, which more closely resembles the one we use today.
Here were the elements known at the time:
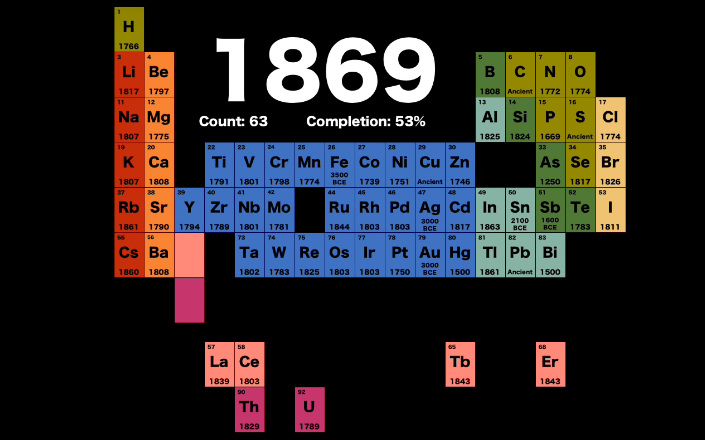
While nowhere near complete, it includes many of the elements that are used in modern life today.
The Final Touches
By the 20th century, chemistry was becoming more formalized, as we knew more about atoms, protons, electrons, neutrons, and so on. This led to the fleshing out of the periodic table as we know it.
By this point, researchers were even creating radioactive, synthetic elements like unununium (Atomic number 111) which is now known as Roentgenium. Like many other late element discoveries, this one is not found in nature and the most common isotope has a half-life of just 100 seconds.
These final discoveries, some of which happened in recent decades, helped bring up the periodic table to its current size: 118 elements.